
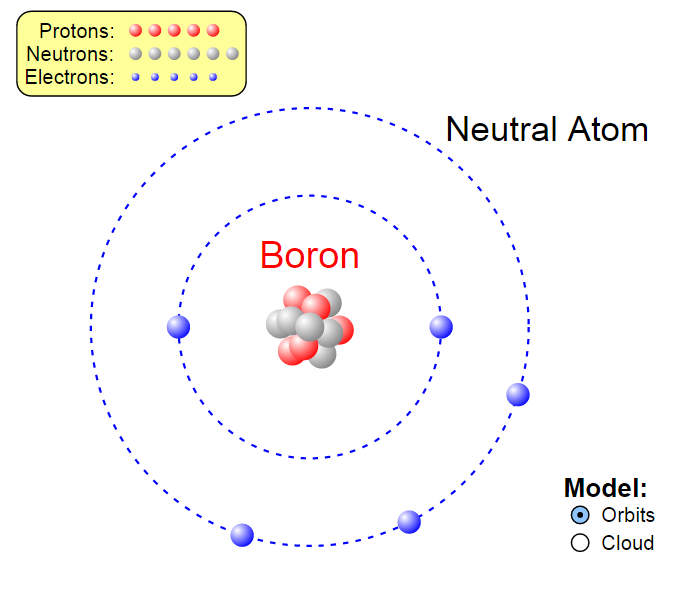

Nevertheless, concerns in relation to reproduction toxicology have arisen and still remain. Studies investigating reproductive toxicity of boron in animal models present results suggesting exposure to boron may induce negative health effects however, due to limited epidemiological studies, these findings cannot be taken as conclusive. Quite the contrary, the findings direct to the belief that boron supplementation could play a preventive role against some diseases. Epidemiological studies so far – which have been limited – have not found any observed endemic disease even in mining areas where boron concentration and exposure are high. Consequently, its extensive use raises the question of whether boron pollution in the environment will constitute a risk for the human being in the future. Day by day, it is becoming increasingly present in different manufacturing areas. Korkmaz, in Encyclopedia of Environmental Health, 2011 Introductionīoron (B) is widespread in nature and is commonly used in industrial fields. Since then, the progress in this field has been extremely slow because of problems associated with mass spectrometric analysis. The first boron isotopic measurement, in fact, was made more than 30 years ago. This mainly is a result of lacking proper analytical technique for B isotopic analysis. It is an underused tracer in earth sciences, in particular when compared with the well established H, O and C stable isotopes. For instance, the boron isotopic compositions in natural waters and show distinctive isotopic compositions in each hydrological reservoir. The unique geochemical characteristics of B, which include high solubility in aqueous fluids, high magmatic incompatibility, and large relative mass difference between two isotopes, make B and δ 11B useful tracers of deep earth fluids and the recycling of subducted materials in convergent margins. In Handbook of Stable Isotope Analytical Techniques, 2009 Publisher Summaryīoron has two natural stable isotopes, 10B and 11B, with an average abundance of approximately 19.9% and 80.1% respectively. While urine serves as the primary pathway for excretion of boron, bile, sweat, and exhaled breath constitute other routes of elimination. The kidneys are the primary site of homeostatic regulation of boron in the body, and at normal dietary or supplemental levels, there is no evidence for boron accumulation over time. Cessation of dietary boron results in a rapid drop in bone boron levels, with a direct relationship observed between ingestion and urinary excretion rates, which accounts for elimination of nearly 100% of the boron load. Boron metabolism in the body is not energetically feasible, with 523 kJ mol −1 energy being required to break boron–oxygen bonds.

An oral dose of boron is readily and completely (approximately 95%) absorbed in humans and appears rapidly in the blood and body tissues. The decisive factor for the degree of liberation of boron from boron-containing topical products is the nature of the vehicle, with water-based vehicles resulting in higher boron levels achieved in blood and urine.
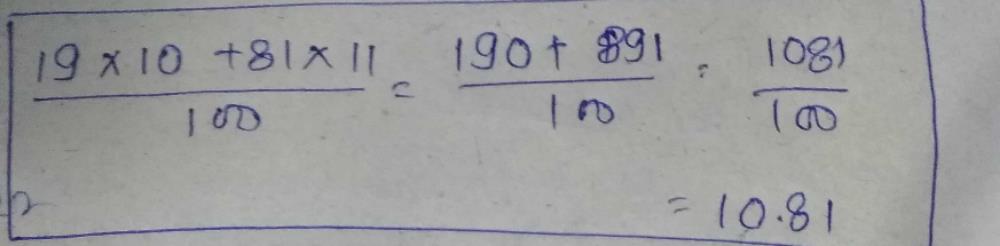
#ATOMIC MASS OF BORON SKIN#
While absorption through intact skin is negligible, it can occur through denuded or irritated skin. Betharia, in Encyclopedia of Toxicology (Third Edition), 2014 Toxicokineticsīoron-containing compounds can be absorbed from both the gastrointestinal and respiratory tracts, as indicated by an increase in levels of boron in the blood, tissues, or urine or by systemic toxic effects observed in the exposed individuals or laboratory animals.


 0 kommentar(er)
0 kommentar(er)
